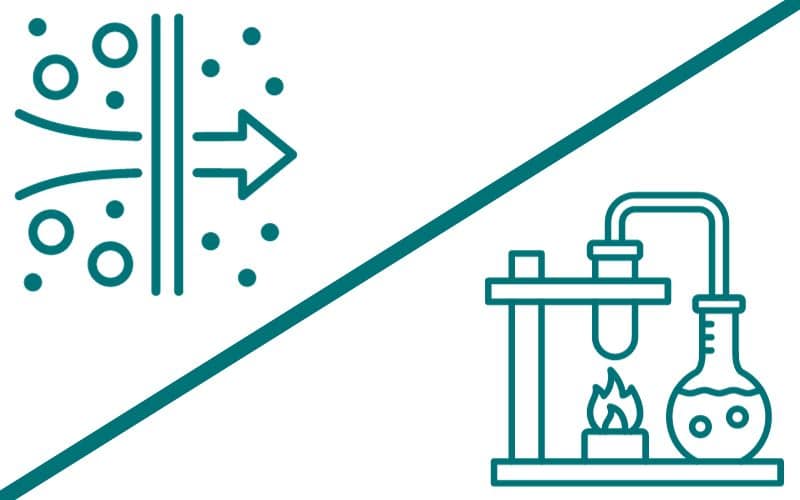
Reverse osmosis (RO) and distilled water are both highly effective water purification methods, each with its own unique process and set of advantages, particularly for household use. Understanding their differences and the specific benefits of reverse osmosis systems is crucial in determining why they might be a better option for the average household.
Reverse Osmosis: Scientific Process
- Principle: Reverse osmosis is based on the principle of osmosis but in reverse. Osmosis is a natural process where water moves across a semi-permeable membrane from a lower-concentration solution to a higher-concentration one. Reverse osmosis applies external pressure to reverse this natural flow.
- Membrane Function: The key component of reverse osmosis is the semi-permeable membrane. This membrane has extremely tiny pores, about 0.0001 microns in size. These pores are small enough to allow water molecules to pass through but block larger molecules and ions, such as salts, minerals, and other impurities.
- Pressure Application: In reverse osmosis, pressure is applied to the water on one side of the membrane. This pressure must be greater than the naturally occurring osmotic pressure in order to reverse the flow of water. When this happens, water molecules are forced through the membrane to the lower concentration side.
- Contaminant Rejection: As water passes through the membrane, contaminants that are too large to fit through the pores are left behind and eventually flushed away. This includes a wide range of contaminants, from bacteria and viruses to chemical impurities like fluoride and nitrates.
Distillation: Scientific Process
- Boiling and Evaporation: Distillation begins with boiling the water. When water is heated to its boiling point, it turns into vapor, leaving most impurities behind in the liquid phase. This is because the impurities generally have higher boiling points than water.
- Condensation: The water vapor is then directed into a cooling chamber. As the vapor cools, it condenses back into liquid form. Since most contaminants do not evaporate along with the water, the condensed liquid is much purer than the original water.
- Separation of Contaminants: The process effectively separates substances based on their boiling points. However, certain chemicals that have lower or similar boiling points to water, like some volatile organic compounds (VOCs), can also vaporize and condense along with the water. This can be addressed with additional purification steps, such as carbon filtration.
- Energy Requirement: The process of distillation requires a significant amount of energy to heat the water to boiling and then cool the vapor back into liquid. This makes it less energy-efficient compared to reverse osmosis.
Key Differences in Processes
- Mechanism: Reverse osmosis relies on a semi-permeable membrane and pressure, whereas distillation relies on phase change (liquid to gas and back to liquid).
- Energy Usage: Reverse osmosis is generally more energy-efficient, using pressure to force water through a membrane, while distillation requires a lot of energy to heat and cool water.
- Contaminant Removal: While both methods are effective at removing a wide range of contaminants, their efficacy varies depending on the type of impurity. Reverse osmosis is highly effective against a wide range of dissolved solids and larger molecules, while distillation is more effective against impurities with higher boiling points than water.
- Time Efficiency: Reverse osmosis can provide purified water more quickly and continuously compared to the slower process of distillation.
- Wastewater Generation: Reverse osmosis generates wastewater in the process, while distillation does not, but consumes more energy.